Our Approach
Our research focuses on using proteomics, immunological, and biochemistry method to investigate the role of innate immune cells in a wide range of diseases including diabetes & obesity, atherosclerosis, infections, and cancer. We took discovery based approach to identify novel changes in protein expression pattern exhibited in altered diseases states, followed by data-driven hypothesis to interrogate…
(can have one image talk about using human, mouse, and in vitro cell approach
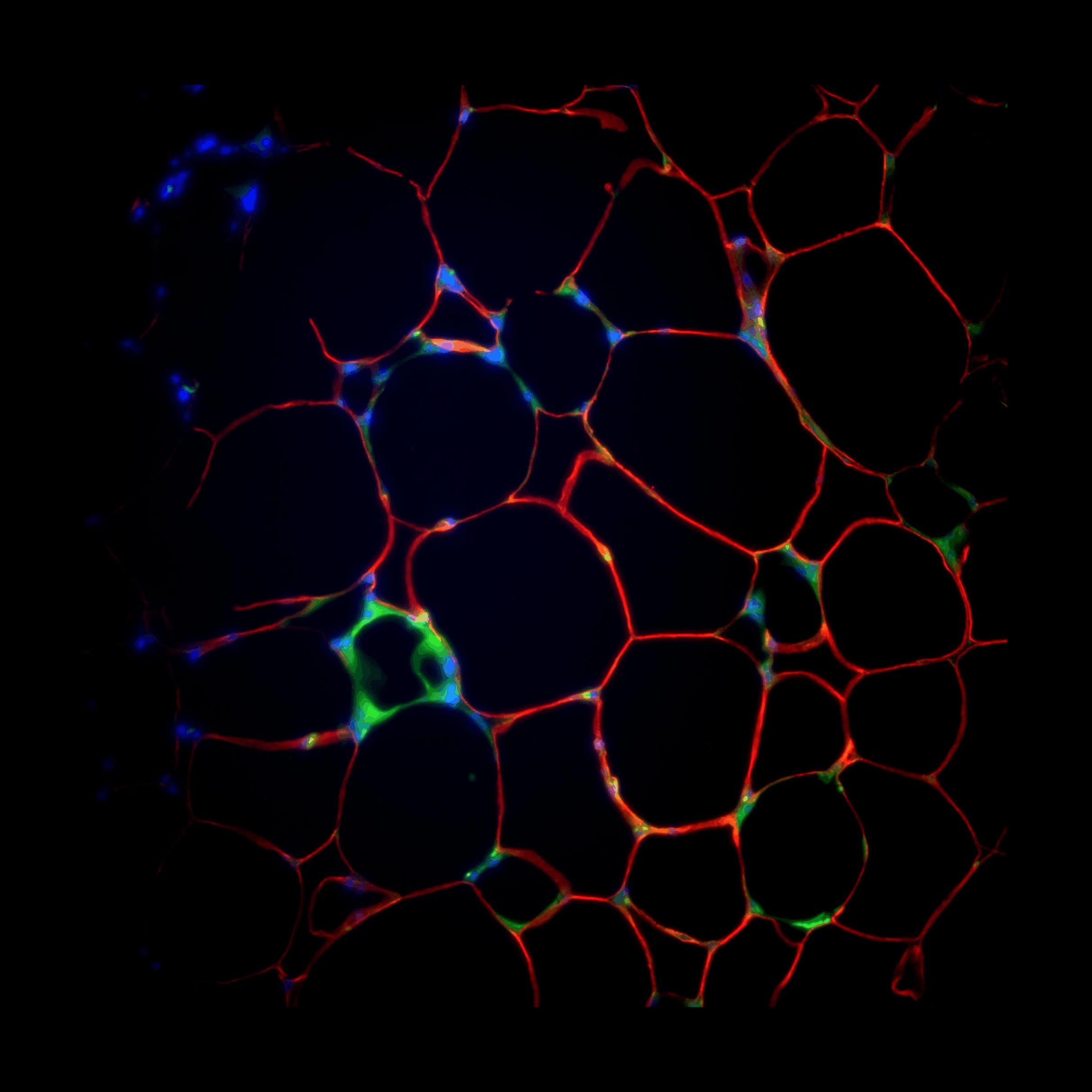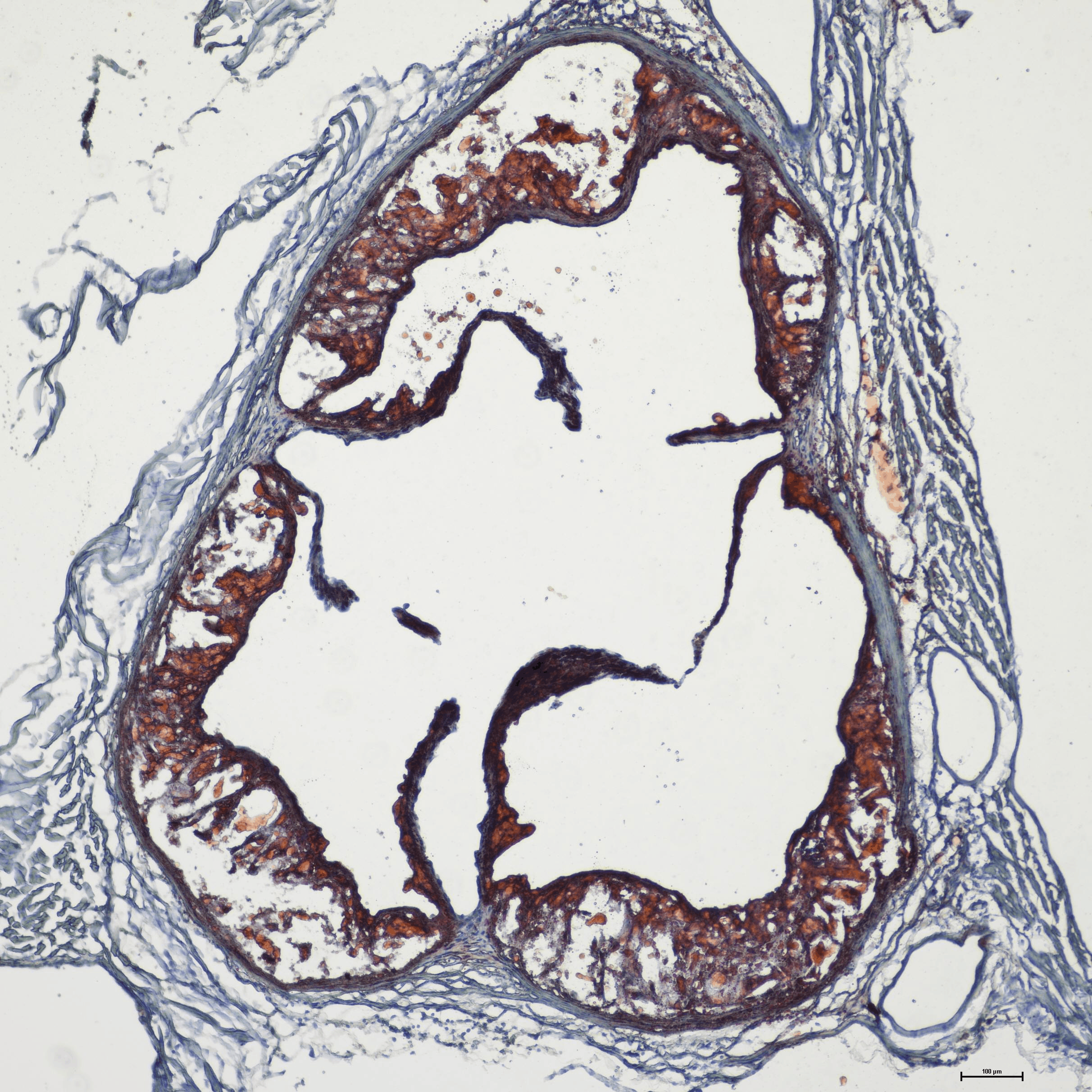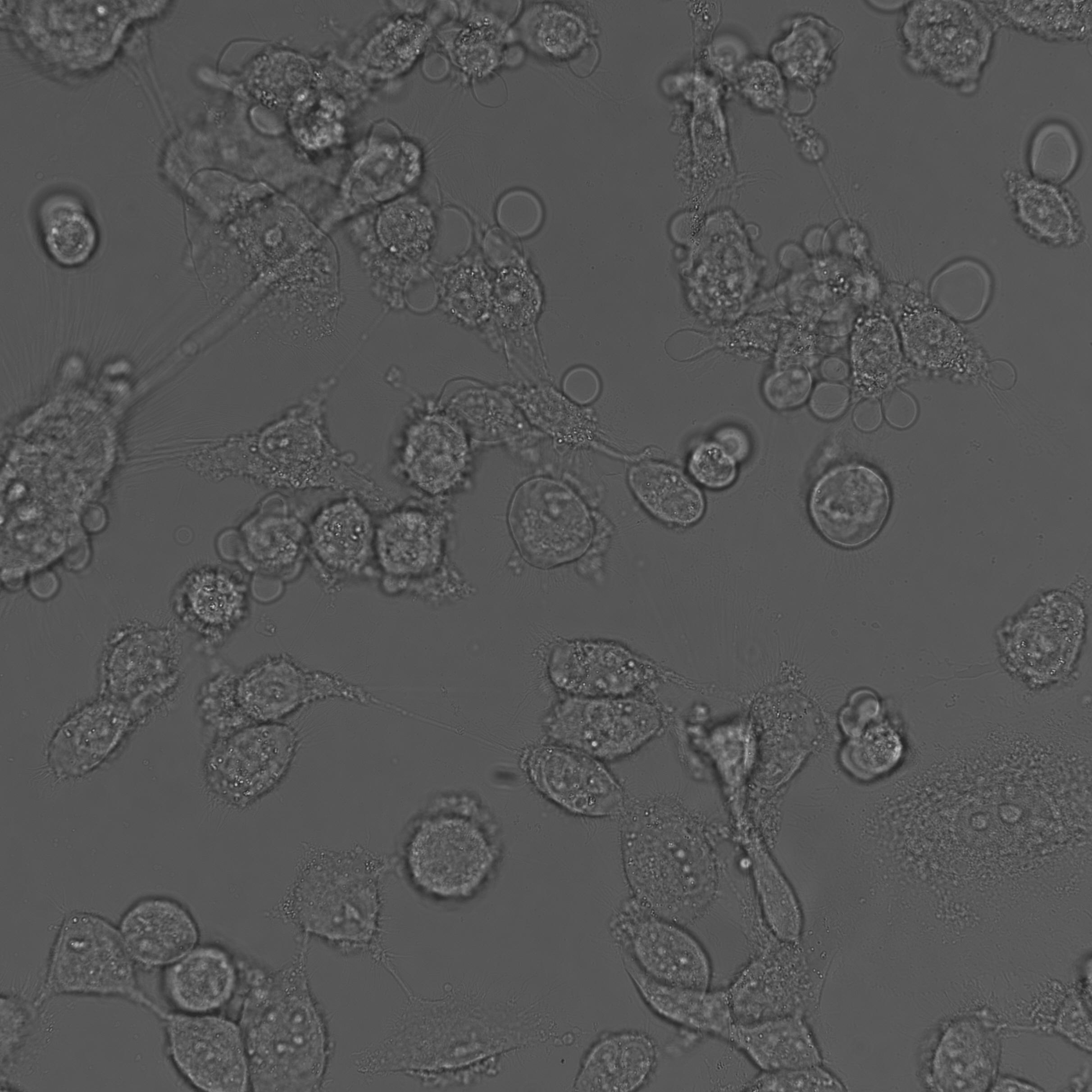
Crown-like Structures
tHE DUAL ROLES OF Metabolically activated MacrophageS IN DIET INDUCED OBESITY.
We found that during obesity, adipose tissue macrophages adopt a metabolically activated (MMe) phenotypes. However, the functionals of MMe are poorly understood. We found that during obesity, adipose tissue macrophages adopt a metabolically activated (MMe) phenotypes. However, the functionals of MMe are poorly understood. We found that during obesity, adipose tissue macrophages adopt a metabolically activated (MMe) phenotypes. However, the functionals of MMe are poorly understood. We found that during obesity, adipose tissue macrophages adopt a metabolically activated (MMe) phenotypes. However, the functionals of MMe are poorly understood. We found that during obesity, adipose tissue macrophages adopt a metabolically activated (MMe) phenotypes. However, the functionals of MMe are poorly understood. We found that during obesity, adipose tissue macrophages adopt a metabolically activated (MMe) phenotypes. However, the functionals of MMe are poorly understood.
Aortic root lesion area
obesity and Insulin Resistance Promote Atherosclerosis through an IFNg-Regulated Macrophage Protein Network.
Type 2 diabetes (T2D) is associated with increased risk for atherosclerosis; however, the mechanisms underlying this relationship are poorly understood. Macrophages, which are activated in T2D and causatively linked to atherogenesis, are an attractive mechanistic link. Here, we use proteomics to show that diet-induced obesity and insulin resistance (obesity/IR) modulate a pro-atherogenic "macrophage-sterol-responsive-network" (MSRN), which, in turn, predisposes macrophages to cholesterol accumulation. We identify IFNγ as the mediator of obesity/IR-induced MSRN dysregulation and increased macrophage cholesterol accumulation and show that obesity/IR primes T cells to increase IFNγ production. Accordingly, myeloid cell-specific deletion of the IFNγ receptor (Ifngr1-/-)
ELANE uPTAKED DYING CANCER CELLS
elane-mediated specific cancer cells killing
Cancer is a collection of diseases driven by variable genetic mutations. Overcoming this genetic variability has stymied development of broad anti-cancer therapies. Our innate immune system evolved to protect against genetically diverse pathogens and limit host toxicity. However, whether/how the innate immune system can be leveraged to produce similar effects in cancer has not been fully elucidated. Here we show that human neutrophils release factors that kill a wide range of cancer cells, but spare non-cancer cells, and identify neutrophil elastase (ELANE) as the driver of this selective killing. We show that ELANE cleaves CD95 to release a death domain-containing proteolytic fragment that is selectively toxic to cancer cells. Intratumorally-delivered ELANE induces cancer cell apoptosis, attenuates tumorigenesis, and promotes CD8+T-cells to attack distant tumors in multiple models. In contrast, ELANE does not produce evident toxicity in tumor-free mice.Our studies suggest that ELANE may overcome cancer cell heterogeneity to potentially function as a broad anti-cancer therapy